
The legacy of
a community committed
Research. Policy. Opportunity. Freedom.
As we celebrate 75 years of progress, we have no intention of slowing down. We’re building on this momentum by:
- Expanding research into gene therapies and muscle regeneration
- Advocating for policies that empower people with neuromuscular disease
- Supporting access to medical care through MDA’s Care Center Network
- Continuing to build and empower a strong, connected community
Scroll down to explore the moments in which we’ve made history—and the impacts that have been felt by millions.

1949
Dr. Ade Milhorat is one of few doctors researching muscular dystrophies worldwide
In 1949, Dr. Ade Milhorat of New York Hospital-Cornell Medical Center was one of only a handful of doctors in the world researching muscular dystrophies. His research work lost funding, and it might well have been the end of his efforts were it not for a friend who also had muscular dystrophy. That friend was Paul Cohen.

1950
The Muscular Dystrophy Association of America is founded
On June 6, 1950, Paul Cohen, a prominent New York businessman with a form of muscular dystrophy (FSH), invited a group of individuals to meet in his Rye, NY office. Each had a personal connection to muscular dystrophy, and the gathering focused on the urgent need to raise funds to advance research seeking treatments and cures for muscular dystrophy. The group formed the Muscular Dystrophy Association of America (MDAA)*, and two months later, MDA made its first research grant to support Dr. Milhorat’s research program.

1952
Dean Martin, Jerry Lewis promote MDA on national radio
During their January 4, 1952 radio program, Martin and Lewis make an appeal for nationwide support of MDA. Later that year, Martin and Lewis were named MDA National Co-Chairmen.
1952
MDA Ambassador Program launched
MDA introduces its first Ambassador Program to raise awareness about muscular dystrophy and other neuromuscular disorders. Over time, the program evolves to highlight the strength and diverse stories of people living with neuromuscular disease in the MDA community.

1952
Five MDA Local Telethons Held in Two Years
Early to recognize the power of television to bolster awareness and raise income for the Association, MDA’s top fundraising priority was the pioneering work of establishing local Telethons featuring a variety of celebrities. Thanks to the help of top stars like Robert Alda, Dick Van Dyke, Captain Video and Virginia Graham, MDA successfully hosted five local Telethons in two years in Cleveland; Washington DC; Atlanta; Grand Rapids; and Madison, Wisconsin.


1953
National Association of Letter Carriers becomes MDA's first national sponsor
MDA Founder Paul Cohen recruits the National Association of Letter Carriers (NALC) as the organization’s first national sponsor. The NALC quickly established a nationwide door-to-door campaign for MDA, the first of which took place on November 25, 1953. The inaugural campaign was heralded by a special two-hour coast to coast television show hosted by Martin and Lewis.

1954
The International Association Resolves to Fight Muscular Dystrophy and "Fills the Boot"
Early in 1954, a group of families affected by muscular dystrophy approached Local 718 fire station in the Boston area to ask professional fire fighters to help fight muscular dystrophy. Responding enthusiastically, the fire fighters took to the streets with their boots in hand to ask greater Boston to make donations that would be used to fight muscular dystrophy. The Fill the Boot campaign was an instant success and on August 19, 1954, the International Association of Fire Fighters (IAFF) membership passed a resolution to support the Association’s fight against muscular dystrophy until treatments and cures are found.

1955
Eleanor Gehrig named MDA's National Campaign Chairperson
After losing her husband, New York Yankees icon Lou Gehrig, to ALS, Eleanor Gehrig made it her mission to help others with the disease. To do so, she partnered with MDA, spearheading advocacy and care efforts that changed the future of life with ALS for good.


1956
Martin and Lewis Local Telethon for MDA at Carnegie Hall
Martin and Lewis co-hosted the 1956 Telethon at New York City’s famed Carnegie Hall and broadcast June 29-30 on Dumont Station WABD (now WABC) in New York, raising some $600,000.

1966
MDA's Legendary Labor Day Telethon Series Begins
The first MDA Jerry Lewis Labor Day Telethon was broadcast in 1966 by a single station, WNEW-TV in New York. The telecast, which resulted in $1,002,114 being pledged, was so successful that MDA selected Labor Day weekend for its future Telethons.


1986
CITGO Joins Forces with MDA
CITGO Petroleum Corporation begins its enduring partnership with the Muscular Dystrophy Association. Over the decades, CITGO employees, marketers, and retailers have supported MDA through volunteer efforts and community engagement, helping to advance critical programs such as MDA Care Centers, summer camps, and research initiatives.

1986
MDA-funded discovery of the DMD gene launches a new era in genetic research and therapies.
In 1986, MDA-funded researcher Dr. Lou Kunkel and his team identified the gene responsible for Duchenne and Becker muscular dystrophy, a landmark discovery that paved the way for targeted research and innovative treatments. This breakthrough marked the beginning of the gene discovery era, transforming scientific understanding of neuromuscular diseases and laying the foundation for advancements in genetic therapies that continue to benefit the neuromuscular community today.

1990
Americans with Disabilities Act (ADA) signed into law
On July 26, 1990, President George H.W. Bush signed into law this landmark civil rights legislation intended to eliminate barriers for people with disabilities. Driven by the advocacy work of MDA and other organizations, this pivotal law represents a critical milestone in advancing the rights and independence of people living with neuromuscular diseases.

1992
Genetic mutations causing myotonic dystrophy type 1 identified
In 1992, MDA supported research leading to the identification of genetic mutations causing myotonic dystrophy type 1 (DM1)
1993
Mutations in PMP22 identified as a causative for CMT1A
In 1993, mutations in PMP22 were identified as a causative for CMT1A (first localized to 17p11.2) by a collaborative group sponsored by MDA.
1995
FDA approves Riluzole, first treatment for ALS
In 1995, the FDA approved riluzole, the first-ever treatment for ALS, offering new hope for people living with the disease. Backed by years of research, including contributions from MDA-supported scientists, riluzole’s approval represented a critical step in ALS treatment, paving the way for further advancements in therapies aimed at improving quality of life and extending survival for people living with ALS.

1996
American Medical Association honors MDA with the Lifetime Achievement Award
In 1996, the American Medical Association honored MDA with the prestigious Lifetime Achievement Award, recognizing decades of pioneering work in neuromuscular disease research, care, and advocacy. This accolade underscores MDA's enduring commitment to leading efforts that have shaped the landscape of neuromuscular medicine.

1998
MDA-funded work leads to three FDA-approved exon skipping drugs for Duchenne muscular dystrophy
In 1998. MDA-funded research contributed to the development of three FDA-approved exon-skipping drugs for Duchenne muscular dystrophy, offering targeted treatment options for specific genetic mutations within the disorder. These therapies marked a significant advancement in Duchenne care, slowing disease progression for eligible individuals and opening doors to further genetic-based approaches.

1999
MDA funds first clinical trial for gene therapy for any type of muscular dystrophy
In 1999, the Muscular Dystrophy Association funded the first clinical trial for gene therapy for any type of muscular dystrophy. This trial was led by Dr. Jerry Mendell and was the first gene therapy trial for muscular dystrophy.

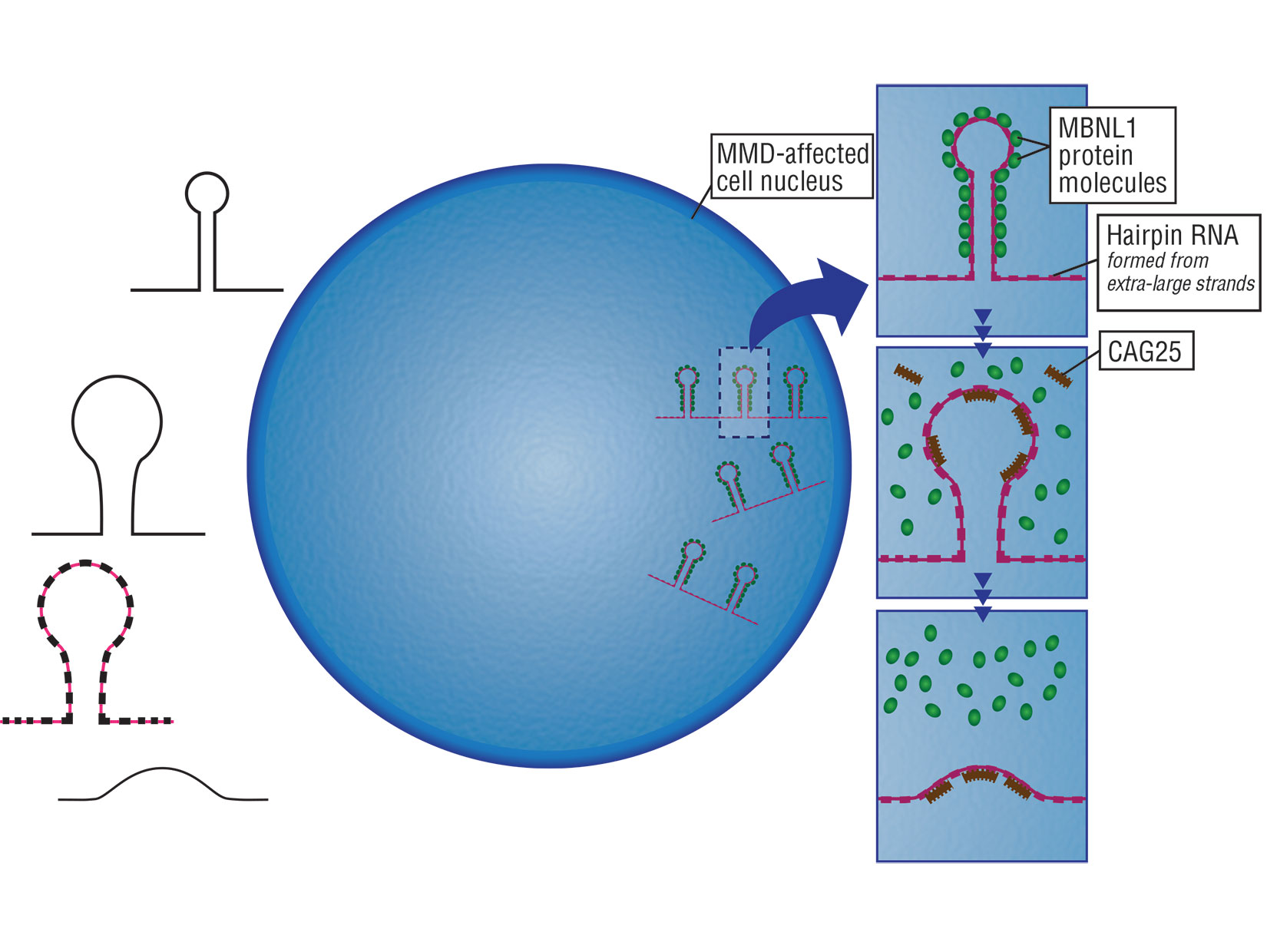


2003
The Centers of Excellence program in muscular dystrophy research is established by NIH
The Centers of Excellence program in muscular dystrophy research was established in 2003 by NIH in honor of the late Senator Paul D. Wellstone of Minnesota.
The Wellstone Centers focus on translating basic research into clinical applications, enhancing diagnostics, and developing new therapies, with an emphasis on collaboration across institutions.
2006
MDA funds the first-ever gene therapy trial for Duchenne muscular dystrophy
In 2006, MDA funded the first-ever gene therapy trial for Duchenne muscular dystrophy, marking a transformative moment in the pursuit of neuromuscular disease treatments. This pioneering trial paveed the way for gene-based approaches aimed at addressing the root causes of Duchenne, opening the door to innovative therapies for a range of neuromuscular diseases.

2006
FDA approves Lumizyme (alglucosidase alfa) for the treatment of Pompe Disease
In 2006, the FDA approved Lumizyme, a groundbreaking enzyme replacement therapy for Pompe disease. The development of the therapy, funded in part by MDA, marked a significant advancement in care, offering new hope for improved quality of life.

2007
MDA funds Dr. Adrian Krainer's antisense therapy research, leading to Spinraza for SMA
In 2007, MDA funded pioneering research by Dr. Adrian Krainer in antisense therapy, leading to a breakthrough in treatment for spinal muscular atrophy (SMA). Dr. Krainer’s work culminates in the development and FDA approval of Spinraza, the first therapy to effectively target the genetic root of SMA, transforming SMA from a terminal diagnosis in young children to a manageable condition.



2015
MDA makes the difficult decision to end its historic Telethon tradition
As families and supporters began looking for new ways to support and get involved with the organization, MDA embraced new opportunities through social media and other digital channels to inspire the nation in support of the fight against neuromuscular diseases.


2015
MDA establishes the MDA Resource Center to provide trusted guidance and support nationwide
In 2015, MDA established the MDA Resource Center as the organization’s national information hub, offering individuals and families a reliable source of information, guidance, and support to navigate life with neuromuscular disease.

2016
FDA approves Exondys 51 (eteplirsen) for the treatment of DMD
After decades of funding Duchenne muscular dystrophy research, the MDA-supported landmark treatment, Exondys 51 (eteplirsen), was approved by the FDA in 2016. This therapy was the first approved drug to treat Duchenne muscular dystrophy (DMD) in patients who have a confirmed mutation of the dystrophin gene amenable to exon 51 skipping.
FDA approves Spinraza (nusinersen) for the treatment of SMA
In 2016, the FDA issued a landmark approval for Spinraza, providing life-changing therapeutic options for indivisuals with SMA. With foundational support from MDA, this treatment offers the potential to slow disease progression and improve motor function.

2017
FDA approves Emflaza (deflazacort) for the treatment of Duchenne Muscular Dystrophy
In 2017, the FDA approved Emflaza, a signature treatment for the most common form of childhood muscular dystrophy, Duchenne, after decades of MDA research, funding, and trials.
FDA approves Radicava (edaravone) for the treatment of ALS
After completing human trials at more than a dozen MDA care centers, the FDA approved Radicava for the treatment of ALS in 2017.
FDA approves Soliris (eculizumab) for the treatment of myasthenia gravis
In 2017, the FDA approved Soliris for the treatment of myasthenia gravis. A first-of-its-kind complement inhibitor drug, this therapeutic strategy has paved the way for new care options for the MDA community, and new paths for MDA-funded research.

2018
Decoding Disease Mechanisms
In 2018, MDA funded grants to Dr. Arthur Burghes (Ohio State) for identifying modifiers of disease and Dr. Rashmi Kothary(Ottawa Hospital Research Institute) to study abnormal metabolism in SMA.

2018
MDA launches the MOVR Data Hub™
In 2018, MDA launched the MOVR (NeuroMuscular ObserVational Research) Data Hub™, a neuromuscular disease data platform that centralizes clinical information from MDA Care Centers across the country. This comprehensive resource empowers researchers, clinicians, and healthcare providers with real-time insights, accelerating progress in understanding disease patterns, treatment outcomes, and patient needs.

2018
Adrian Krainer, PhD, wins Breakthrough Prize for developing Spinraza, an SMA therapy
In 2018, MDA researcher Adrian Krainer, PhD, received the Breakthrough Prize in Life Sciences for the development of an effective antisense oligonucleotide therapy (Spinraza) for children with SMA.Dr. Krainer’s work with Spinraza underscores the power of targeted genetic therapies and sets a precedent for future innovations in treating neuromuscular diseases.
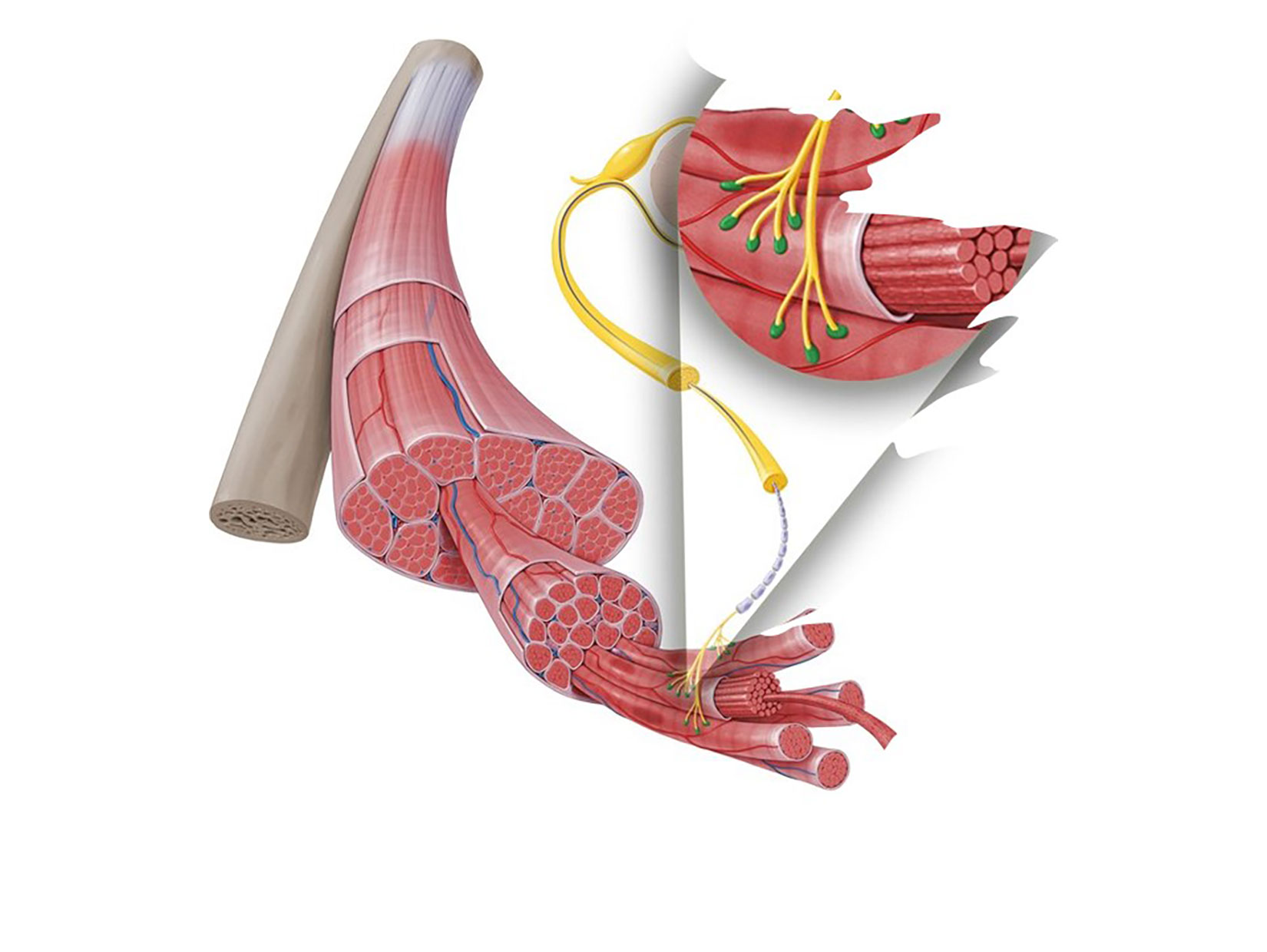
2018/2019
FDA approves Firdapse/Ruzurgi (amifampridine) for the treatment of Lambert-Eaton Myasthenic Syndrome (LEMS)
As the first treatment for adults with Lambert-Eaton Myasthenic Syndrome (LEMS), the 2018 FDA approval of MDA-supported treatment Firdapse marked a significant step forward in addressing the needs of individuals with LEMS by providing a dedicated therapy to improve muscle strength and quality of life.
2019
FDA approves Zolgensma (onasemnogenebeparvovec-xioi) for the treatment of SMA
In 2019, the FDA approved Zolgensma, a first-of-its-kind, one-time gene therapy that transformed the treatment landscape for SMA. This groundbreaking gene therapy was made possible through the contributions of countless MDA researchers.

2019
FDA approves Vyondys 53 (golodirsen) for the treatment of Duchenne muscular dystrophy
In 2019, the FDA approved Vyondys 53 for the treatment of Duchenne muscular dystrophy. The second ever exon-skipping, disease-modifying drug for DMD, this MDA-supported treatment builds on the momentum created by eteplirsen in developing effective solutions for the challenges of Duchenne.

2019
MDA Hosts First Clinical and Scientific Conference
In 2019, MDA united its previously separate Clinical and Scientific Conferences into one premier event, bringing together the world’s leading experts in neuromuscular medicine and research. This decision fostered interdisciplinary collaboration, facilitating the exchange of ideas between clinicians, researchers, and industry leaders. The combined conference has since become a prestigious convening of experts, advancing innovations in care, sharing groundbreaking research, and accelerating progress in the neuromuscular field.

2020
FDA approves Viltepso (viltolarsen) to treat Duchenne muscular dystrophy
In 2020, the FDA approved the third-ever, exon-skipping treatment, Viltepso, for the fight against DMD. Supported by MDA, this breakthrough builds upon the successes of eteplirsen and golodirsen to create more meaningful treatment options for those living with Duchenne.
FDA approves Evrysdi (risdiplam) to treat spinal muscular atrophy
In 2020, the FDA approved Evrysdi, the third-ever disease modifying therapy approved to treat spinal muscular atrophy. As the leading genetic cause of infant death, this SMA treatment has had a resounding effect on people throughout the MDA community.

2020
Congress passes the ALS Disability Insurance Access Act
In 2020, Congress passed the ALS Disability Insurance Access Act, helping address the urgent needs of the ALS community by eliminating the benefit waiting period and ensuring that individuals with ALS can access SSDI benefits immediately after approval, to receive timely assistance for medical expenses, adaptive equipment, and other essential needs. In partnership with several ALS organizations, MDA's advocacy work helped bring about this landmark legislation, furthering support for individuals and families during a challenging time.

2020
MDA launches Let's Play
In 2020, MDA introduced the Let’s Play program, a unique initiative designed to build an inclusive community for gamers living with neuromuscular diseases. Through adaptive gaming technology, tournaments, and social connections, Let’s Play empowers individuals to participate in gaming experiences regardless of physical limitations.

2020
MDA introduces MDA Virtual Summer Camp
In response to the COVID-19 pandemic, MDA reimagined its beloved Summer Camp by creating a virtual experience for kids and teens living with neuromuscular diseases. The Virtual Summer Camp brought the spirit of connection, fun, and independence directly into campers' homes through interactive activities, games, and shared experiences, ensuring the MDA Summer Camp tradition continued to provide meaningful opportunities for friendship and engagement.

2021
FDA approves Vyvgart (efgartigimod alfa-fcab) to treat generalized myasthenia gravis.
In 2021, the FDA approved Vyvgart as a treatment for generalized myasthenia gravis. Replacing time-consuming infusions with a simple, at-home shot, this MDA-supported treatment creates new hope, new freedom, and new possibilities for those living with gMG.
FDA approves Amondys 45 (casimersen) to treat Duchenne muscular dystrophy
The FDA’s decision to approve Amondys 45 highlights the importance of years of commitment to supporting and funding breakthrough research by MDA and others into gene identification and unlocking the cause of DMD.
FDA approves Octagam 10% [Immune Globulin Intraveneous (Human)] to treat adult dermatomyositis
The FDA’s approval of Octapharma’s Octagam 10% [Immune Globulin Intravenous (Human)] for adults with dermatomyositis marked a meaningful step forward in treating inflammatory myopathies. MDA’s longstanding commitment to funding key research on muscle diseases helped build the knowledge base that makes treatments like Octagam 10 possible.
FDA approves Nexviazyme (avalglucosidase alfa-ngpt) to treat late-onset Pompe disease
The 2021 approval of Nexviazyme by the FDA provided new treatment options for people and families living with Pompe disease. An alternative therapy to the MDA-supported Myozyme, this approval marked an important expansion of drug options to improve lives within the Pompe and MDA communities.

2021
Congress passes the ACT for ALS
In 2021, Congress passed the ACT for ALS, a landmark piece of legislation that accelerates access to urgently needed therapies for the ALS and neuromuscular disease communities. Supported by advocacy from MDA and allies, this Act establishes new pathways to fast-track experimental treatments and expands federal funding for ALS research, bringing hope to thousands of individuals and families facing these progressive diseases.

2021
MDA establishes the MDA Mentorship Program
In 2021, MDA launched the MDA Mentorship Program to support youth and young adults with neuromuscular disease as they navigate higher education and career pathways. This unique program pairs participants with mentors who provide guidance, share insights, and foster connections that help young adults pursue their academic and professional goals with confidence.

2022
FDA approves Ultomiris (ravulizumab-cwvz) to treat generalized myasthenia gravis
The 2022 FDA approval of Ultomiris for treating generalized myasthenia gravis welcomed new therapeutic options for those living with the disease. Studied across 36 MDA Care Centers, this drug and its approval signaled another major step forward in the fight against gMG.
FDA approves Relyvrio (sodium phenylbutyrate/taurursodiol) to treat ALS
In 2022, the FDA approval of Relyvrio marked the fourth approved therapy to treat ALS and the first to target its genetic cause. Developed with substantial support by MDA and other organizations, this treatment is a realization of the hope that genetic medicines could be effective for ALS patients and their families.

2022
MDA introduces the Quest Media Lifestyle platform
In 2022, MDA introduced the Quest Media Lifestyle platform, a comprehensive resource designed to enhance the lives of individuals and families in the disability community. Through engaging articles, expert insights, personal stories, and practical advice, Quest Media offers readers a supportive space to explore topics ranging from health and wellness to accessible technology, education, and career growth.

2022
MDA Advocacy Collaboration Grant program launched
Established in 2022, the Muscular Dystrophy Association Advocacy Collaboration Grant Program funds public policy and advocacy projects that benefit the neuromuscular disease community, supporting initiatives such as non-partisan advocacy campaigns, research projects, stakeholder meetings, and grassroots advocate training.

2022
The Centers for Medicare and Medicaid Services creates new diagnostic codes for LGMD
In 2022, the Centers for Medicare and Medicaid services created new diagnostic codes for limb-girdle muscular dystrophy (LGMD), potentially shortening the diagnostic odyssey that patients face. Lobbied for by MDA and a number of LGMD organizations, these codes will help provide more precise medical care, improve clinical trials, and increase future access to targeted treatments.

2022
Congress passes the FDA User Fee Reauthorization Act
In 2022, the FDA User Fee Reauthorization Act passed Congress, clearing the way for an acceleration of treatment and cure development for rare diseases. This MDA-supported legislation included a suite of incredibly important programs and initiatives including expedited regulatory review, investment in gene and cell therapies, and more.

2023
FDA approves Agamree (vamorolone) to treat Duchenne muscular dystrophy
In 2023, Agamree, a novel therapy supported by MDA Venture Philanthropy, was approved by the FDA for the treatment of DMD.
FDA approves Pombiliti™ (cipaglucosidase alfa-atga) + Opfolda™ (miglustat) 65mg capsules for adults living with late-onset Pompe disease
This approval marked a new chapter in expanding treatment options for Pompe disease. MDA’s funding of foundational research at Duke University contributed to the development of Myozyme, the first approved Pompe therapy.
FDA approves Rystiggo (rozanolixizumab-noli) to treat generalized myasthenia gravis
Rystiggo (rozanolixizumab-noli) is used to treat generalized myasthenia gravis in adults who are anti-acetylcholine receptor- or anti-muscle-specific tyrosine kinase antibody-positive.
FDA approves Qalsody (tofersen) to treat ALS
Qalsody is approved for use for adults with an ALS SOD1 mutation.
FDA approves Skyclaris (omaveloxolone) to treat Friederich's ataxia
Clinical trials of Skyclarys took place at MDA Care Center Network locations including UCLA, University of Florida Neurology, Emory University Hospital, The Ohio State University, and Children's Hospital of Philadelphia.

2023
MDA Kickstart Program launched
In 2023, MDA introduced the Kickstart Program to accelerate the development of therapies for ultra-rare neuromuscular diseases. By providing critical funding and resources to early-stage projects, Kickstart bridges the gap between basic research and clinical application, paving the way for innovative treatments by fostering collaboration among researchers.

2023
MDA creates the Gene Therapy Support Network
In 2023, MDA launched the Gene Therapy Support Network to equip individuals and families with essential information and support as groundbreaking gene therapies emerge for neuromuscular diseases. This first-of-its-kind initiative offers up-to-date resources, expert guidance, and a dedicated support system to help families navigate the complex world of gene therapy.
MDA establishes MDA Connect
In 2023, MDA introduced the MDA Connect program, offering individuals and families affected by neuromuscular diseases personalized, one-on-one video consultations with MDA Specialists. This program provides convenient access to expert guidance on navigating care within MDA’s Care Center Network, locating resources, and learning about MDA programs and engagement opportunities.

2024
A new era of treatment for neuromuscular diseases emerges
In 2024, a new era of treatments emerged with more than 20 FDA-approved therapies now available for neuromuscular diseases—including the first ever treatments for Duchenne muscular dystrophy and spinal muscular atrophy—thanks in part to research supported by MDA
FDA approves Duvyzat (givinostat) to treat Duchenne muscular dystrophy
In 2024, MDA's funding of foundational research led to the FDA approval of Duvyzat, a drug for treating DMD in people 6 years and older.

2024
MDA Gene Therapy Support Network honored at 2024 Advanced Therapies Awards
MDA’s Gene Therapy Support Network received honors at the 2024 Patient Advocacy Award for Non-Profits at the Advanced Therapies Awards.

2024
Congress Passes Air Travel Accessibility Reforms
Due to the dedicated efforts of advocates and the MDA community, Congress passed landmark air travel accessibility reforms within the FAA Reauthorization Act of 2024. This historic legislation mandated critical improvements in air travel standards to ensure safe, dignified, and accessible travel for persons with disabilities. Additionally, it addressed long-standing barriers faced by people with mobility impairments, setting new standards for seating, boarding procedures, and wheelchair handling, among other areas.

2024
All 50 states and DC implement newborn screening for SMA
Thanks in part to MDA’s strong advocacy efforts, all 50 states and Washington, DC, implemented newborn screening for spinal muscular atrophy (SMA) in 2024, making early diagnosis and intervention possible for every newborn with this condition.

2024
MDA College Scholarship Program Established
In 2024, MDA introduced a college scholarship program designed to empower young people with neuromuscular disease as they pursue higher education and career training. This program provides financial support to students, helping to alleviate barriers to educational opportunities and open pathways to personal and professional growth.
Join the Movement
Join us in driving the next 75 years of progress in neuromuscular research, care, and advocacy. Together, we can build a future of greater freedom, hope, and possibility for people living with neuromuscular disease.



